Rocco Casagrande Chosen to Serve on the National Academies Standing Committee on Biotechnology and National Security Needs

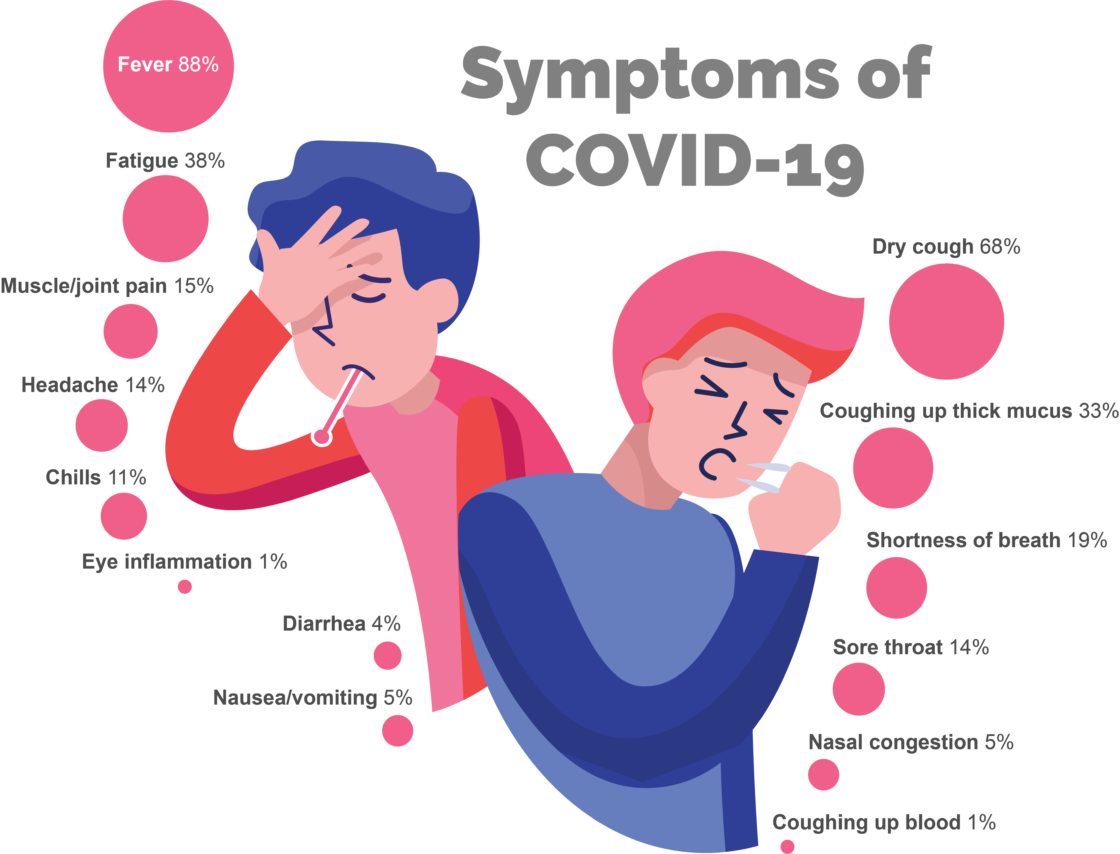
The National Academies of Sciences, Engineering, and Medicine recently accepted Dr. Casagrande as a member of their new standing committee on biotechnology and national security needs. This committee will enable the national security community to identify advanced biotechnology capabilities, discuss potential barriers or challenges to adoption, and build partnerships between the national security stakeholders and biotechnology experts. The standing committee will explore multiple topics including the applicability of biotechnologies for addressing current or anticipated needs of U.S. national security agencies and the factors, such as workforce, infrastructure, policy, and security, needed to enable technology transition to the national security community. Additionally, the committee will examine the early-stage biotechnology and life science research that demonstrates promising scientific or technical capabilities for addressing national security needs and the innovation ecosystem needed to translate early-stage research into practical application.
- GRYPHON PERSONNEL | Dr. Rocco Casagrande
- LEARN MORE | National Academies of Sciences, Engineering, and Medicine