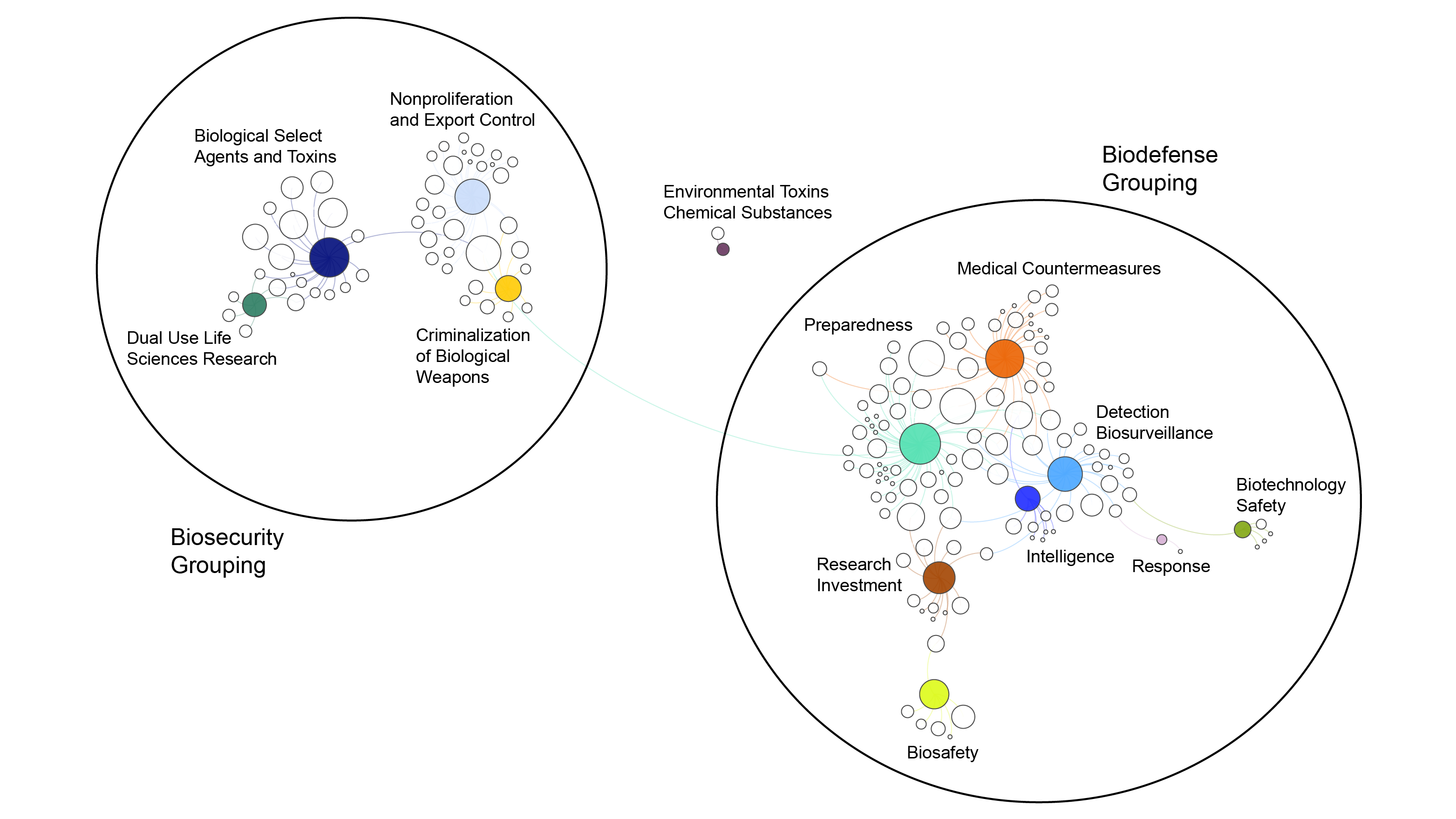
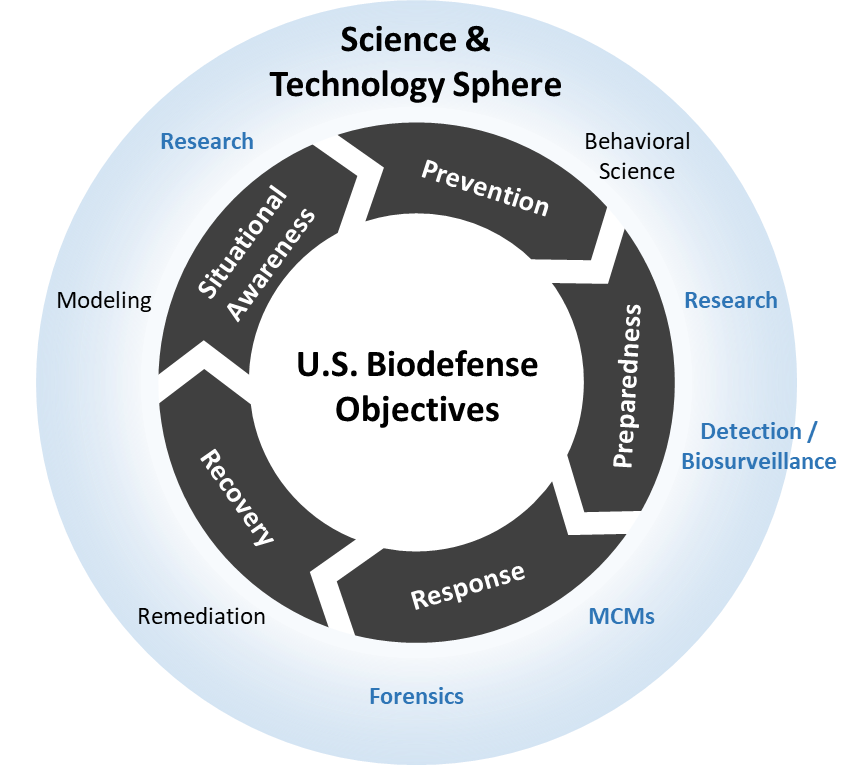
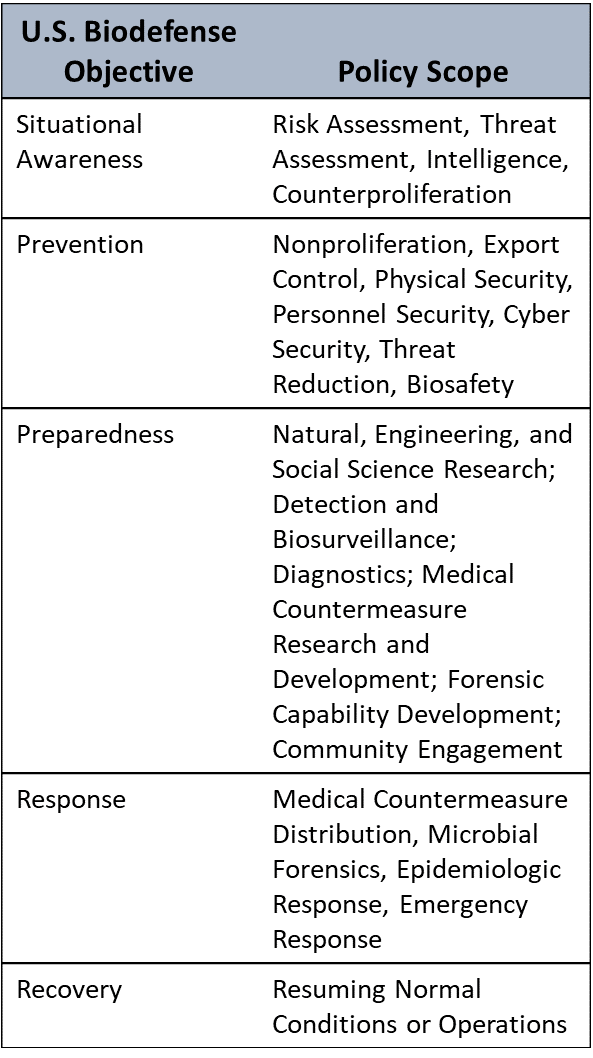
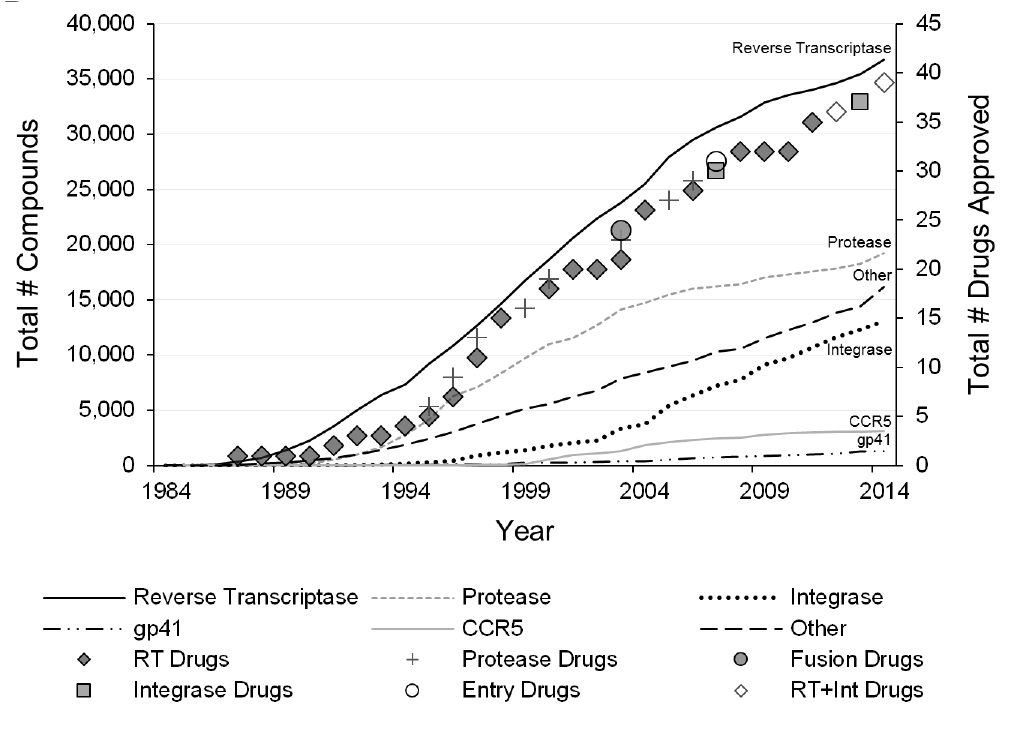
Estimating Risk of Hematopoietic Acute Radiation Syndrome in Children

Children are known to be more radiation sensitive than adults, but insufficient data are available to quantify this increased sensitivity. This paper develops ED50 for hemapoietic injury in children from radiation, allowing improved pediatric casualty estimates.
- PUBLICATION | Learn more here
- GRYPHON STAFF | Louise Sumner, Grace Adams, Dr. Rocco Casagrande (PI)
- PARTNER | Battelle
- CLIENT | Department of Homeland Security S&T